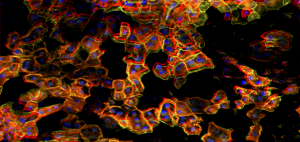
The stiffness of a tissue reflects its cell-level properties. For instance, it is well known that increased stiffness can be an indicator a rapidly growing cancer in a tissue. At UPenn, I am using simulations and theory, supported by experiments with the Penn Science of Oncology Center, to develop the biophysical understanding of tissue mechanics.
On short time scales, biological tissues have the mechanical properties of polymer networks embedded with cells and fluid. This is a complicated composite from a materials physics perspective. Experiments study simplified tissues, with cells alone with no extracellular matrix, or of polymer networks with embedded cell-like particles. A long-term goal is to increase the utility of stiffness in characterizing the microscopic structure of the tissue.